Session Eleven: Medication Trials
This episode is a live recording of the eleventh session of #UnblindingResearch held in DREEAM 16th January 2019. The group work has been removed for the sake of brevity.
This session of #UnblindingResearch looks at the phases of medication trials, the placebo effect and how medicines and medical devices are kept safe. Here are the slides for this session (p cubed of course), you can move between slides by clicking on the side of the picture or using the arrow keys.
Obviously we want to make sure that any medication we give our patients is safe to use. This means we need to trial any possible new drug before it gets a licence for use. However we have to make sure that any trial is conducted in as safe and methodical a way as possible.
The Placebo Effect
As discussed in previous sessions no trial is ever perfect and there is always a chance that any observed effect is due to chance alone. Another issue we face in any clinical trial is the placebo effect. Placebo is Latin for ‘I will please’ and the placebo effect is a well recognised effect in Medicine as this article in Nature from September 2018 demonstrates. In medical trials a placebo is any intervention which is known not to have any therapeutic value (such as a sugar pill.) As discussed in previous sessions placebos are very often used in randomised control trials as a comparison against a potential new treatment. Often the patient is blinded as to whether they have had the new intervention or a placebo. Any measured response to the placebo is called the placebo response. The difference between the response to placebo and the response to no intervention is the placebo effect.
The Phases of Medication Trials
The first stage of any medication trial will begin with a review of previous literature before non-human studies investigating the pharmacokinetics and safety of the potential new drug. This is the pre-clinical stage. There then may be a small study in human subjects using very small doses of the drug to investigate the pharmacokinetics in humans. This step is known as ‘Phase 0’ and very often doesn’t actually take place.
Phase I
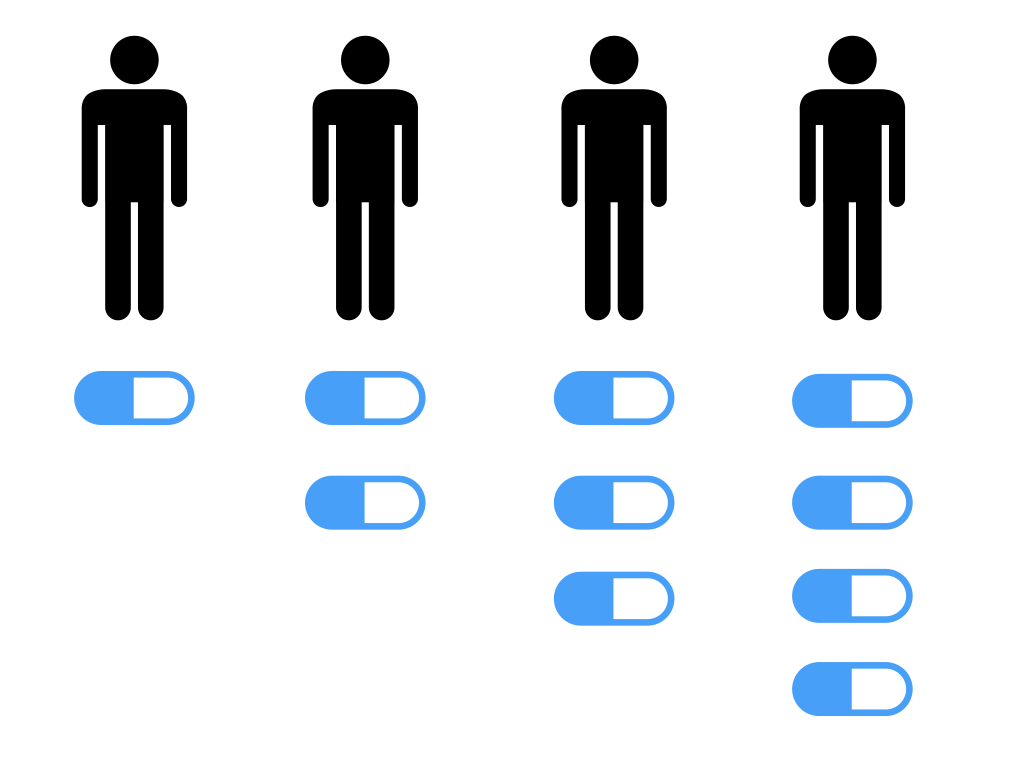
Phase I involves a small study in human volunteers and is interested in drug safety. Low but ascending doses of the drug are given to the volunteers. This gives us an idea of dose ranging and any potential side effects or toxicity. Phase I will involve healthy volunteers usually but for cancer drugs may involve patients with cancer.
Phase II
If a drug passes Phase I we’re now interested in whether the drug has a therapeutic effect in ideal conditions. This is known as the drug’s efficacy. Phase II may be divided into two: Phase IIa and IIb. Phase IIa is interested in looking at whether the drug shows therapeutic effect in ideal conditions (such as whether a potential new tumour drug actually shrinks tumour cells.) IIb looks at finding an optimum dose range for the drug in ideal conditions balancing therapeutic effect with side effect/toxicity profile. Volunteers will have the specific disease we are interested in and we’ll usually recruit a few hundred.
Phase III
If the drug gets through Phase II we’re now interested in how it works in real life conditions such as those a patient will encounter. This is known as the drug’s effectiveness. Phase III will involve a larger sample of volunteers with a specific disease up to a few thousand in number.
Phase IV
By this point the drug has been proven to work and be safe and has been approved for use and so granted a licence. Phase IV trials involve looking at the long-term effects of the drug and more about its side effect profile. There is no limit to Phase IV as the drug is being used in the wide community. The Medicines and Healthcare products Regulatory Agency (MHRA) is an executive agency of the Department of Health and Social Care which is responsible for ensuring the safety of medicines and medical devices. The Yellow Card Scheme (available as a smartphone application) collects data related to safety. This includes suspected adverse drug reactions and defective or counterfeit medications.
Cancer Research UK have a really good page on the phases of clinical trials here.
Here is the #TakeVisually for this episode:
Remember the next session: